Act As A Catalyst Meaning
Goad is a common word that you might encounter while studying chemistry peculiarly while learning about chemic reactions. While some of the chemical reactions occur rapidly, some accept a long time and require actress materials or effort. This is where a goad comes in. What is the meaning of catalyst? We volition discover out in this lesson.
Tabular array of Content
- Types of Catalysts
- Catalysis
- Heterogeneous Catalysis
- Homogeneous Catalysis
- Autocatalysis
- Frequently Asked Questions
What is Catalyst in Chemistry?
In Chemistry, catalysts are defined every bit those substances which alter the charge per unit of reaction by irresolute the path of reaction. Most of the time a catalyst is used to speed upward or increase the rate of the reaction. Still, if we become to a deeper level, catalysts are used to break or rebuild the chemical bonds betwixt the atoms which are present in the molecules of different elements or compounds. In essence, catalysts encourage molecules to react and brand the whole reaction process easier and efficient.
Some of the important characteristic features of catalysts are,
- A goad does not initiate a chemic reaction.
- A catalyst does not be consumed in the reaction.
- Catalysts tend to react with reactants to form intermediates and at the aforementioned time facilitate the production of the final reaction product. After the whole process, a goad can regenerate.
A catalyst can be either solid, liquid or gaseous catalysts. Some of the solid catalysts include metals or their oxides, including sulphides, and halides. Semi-metallic elements such as boron, aluminum, and silicon are also used as catalysts. Likewise, liquid and gaseous elements which are in pure form are used as catalysts. Sometimes, these elements are also used along with suitable solvents or carriers.
The reaction which involves a goad in their system is known as a catalytic reaction. In other words, catalytic action is a chemic reaction betwixt the catalyst and a reactant. This results in the germination of chemical intermediates that tin can farther react quite readily with each other or with another reactant to form a production. All the same, when the reaction betwixt the chemical intermediates and the reactants occurs or takes place the catalyst is regenerated.
The reaction modes between the catalysts and the reactants ordinarily tend to vary widely and in the case of solid catalysts, it is more circuitous. Reactions tin can be acid-base reactions, oxidation-reduction reactions, coordination complexes germination likewise as production of free radicals. For solid catalysts, the reaction mechanism is greatly influenced by surface properties and electronic or crystal structures. Some types of solid catalysts such every bit polyfunctional catalysts can have several reaction modes with the reactants.
As well Read: Chemic Kinetics
Cursory History
If we wait at the general meaning of catalyst it is anything that increases the charge per unit of a process. Catalyst is a term derived from Greek καταλύειν, significant "to annul," or "to unite," or "to pick upwardly." Meanwhile, the concept of catalysis was first researched by pharmacist Elizabeth Fulhame and it was described in her book in the year 1794. This book content was based on her work in oxidation-reduction experiments.
The first chemical reaction in organic chemistry that utilized a catalyst was studied in 1811 by Gottlieb Kirchhoff who was a Russian chemist of German origin. The term catalysis was later used by a Swedish chemist named Jöns Jakob Berzelius in 1835 to describe reactions that were sped up by certain substances. The substances further remained unchanged after the reaction.
Types of Catalysts With Examples
There are several types of catalysts that can be used depending on the demand or requirement of the chemical reaction. They are equally follows;
Positive Catalysts
Catalysts that increment the charge per unit of a chemic reaction are positive catalysts. It increases the rate of reaction by lowering the activation energy barriers such that a large number of reaction molecules are converted into products, thereby the percentage of yield of products increases.
Positive catalyst example: In the preparation of NH3 past Haber's process Atomic number 26 oxide acts as a positive goad and increases the yield of ammonia in spite of less reaction of Nitrogen.
Negative Catalysts
Catalysts that decrease the rate of reaction and negative catalyst. It decreases the rate of reaction by increasing the activation energy barrier which decreases the number of reactant molecules to transform into products and hence the rate of reaction decreases.
Negative goad example: Decomposition of Hydrogen peroxide into h2o and oxygen is retarded past using Acetanilide, this acts as a negative catalyst to subtract the charge per unit of decomposition of hydrogen peroxide.
Promoter or Accelerators
A substance that increases the catalyst activeness is known every bit a Promoter or accelerator.
Example: In Haber's process molybdenum or a mixture of potassium and Aluminium oxides act as Promoters.
Catalyst Poisons or Inhibitors
Substances that decrease the goad activity are known as catalyst poisons or inhibitors.
Case: In the hydrogenation of alkyne to an alkene, catalyst palladium is poisoned with barium sulphate in quinolone solution and the reaction is stopped at alkene level. The catalyst is known as Lindler's catalyst.
Units
The derived SI unit for measuring the catalytic activity of a catalyst is "katal". It is further quantified in moles per 2nd. If nosotros were to draw the productivity of a goad it tin be defined past the turnover number (or TON). Catalytic activity tin be described by the turn over frequency (TOF) which is TON per time unit. Besides, the enzyme unit is its biochemical equivalent.
Also Read: Enzyme Goad
Catalysis
When a goad is used to increase the charge per unit of a chemic reaction this phenomenon is known as catalysis.
What are the Types of Catalysis?
On the footing of nature and the physical state of substance employed in the chemical reaction, catalysis is of iii types;
- Homogeneous catalysis
- Heterogeneous catalysis
- Autocatalysis
Heterogeneous Catalysis
In this type of catalysis, the reacting substances in a reaction and catalyst employed in that reaction are non in the same country of affair.
Examples 1: Preparation of Ammonia past Haber's process.
Pure and dry Nitrogen and Hydrogen gases in one : iii ratio are passed through a compressor where loftier pressure level of 200 – 30 atmosphere is maintained. In this procedure, Fe oxide is used every bit a catalyst. It is solid oxide employed in a process where the reactants are in a gaseous state. The Nitrogen (one thousand) reacts with hydrogen (1000) to course Ammonia (1000), in the pressure of Iron oxide solid thus information technology is heterogeneous catalysis
Case 2: Manufacture of sulphuric acid past Contact procedure.
In this procedure, the oxidation of sulphur dioxide is a major step. In this oxidation, sulphur dioxide is a gas and oxygen is a gas while vanadium pentoxide is a solid catalyst. In this process, reactants and catalysts are in unlike states of matter.
Mechanism of Heterogeneous Goad
Heterogeneous catalysis involves both adsorption as well as intermediate compound formation. The reactant molecule gets adsorbed on the activation centre of the surface of the catalyst. These combine to form an activated complex which is an intermediate compound. This chemical compound decomposes to give products.
Every bit before long as the products are formed these get desorbed from the surface without whatever lapse in fourth dimension. The heterogeneous catalysis involves initially adsorption of reactants on the surface of catalyst, Intermediate chemical compound formation, dissociating into a product.
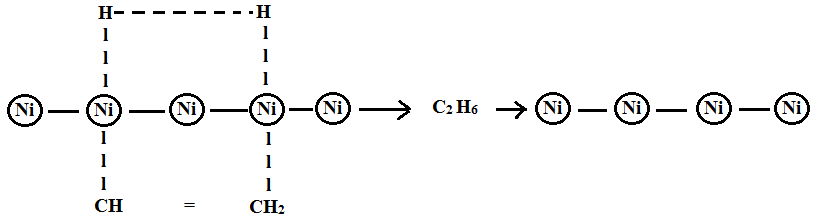
Example: Hydrogenation of ethene into ethane on the surface of the nickel.
- Ether and hydrogen molecules are adsorbed on the surface of the goad.
- Hydrogen occupies most of the activation centre and is known equally apoplexy.
- Ethane molecules attack at their double bail region to grade an activated complex.
- Ether reacts with active hydrogen to grade ethane.
- This ethane gets desorbed on the surface of the goad.
Electrocatalysts
In electrochemistry especially when we are dealing with fuel jail cell engineering science, several types of metallic-containing catalysts are used. These catalysts accept i primary part which is to heighten the rates of the one-half-reactions that occur in a fuel cell. A popular electrocatalyst used in a fuel cell is nearly of the time based upon nanoparticles of platinum. Now, these are supported on slightly larger carbon particles. Every bit this catalyst comes in contact with one of the electrodes in a fuel jail cell, platinum increases the rate of oxygen reduction either to h2o or hydroxide (also hydrogen peroxide).
Homogeneous Catalysis
The catalysis in which the catalyst employed in the reaction and the reactants are in the same state of affair, that process is referred to as homogeneous catalysis. In the same step equally the reactants, homogeneous catalysts work. Usually, homogeneous catalysts with substrates are dissolved in a solvent. The effect of H+ on the esterification of carboxylic acids, such as the germination of methyl acetate from acetic acrid and methanol, is 1 example of homogeneous catalysis. Hydroformylation, hydrosilylation, hydrocyanation involve high-volume processes requiring a homogeneous catalyst. Homogeneous catalysis is frequently synonymous with organometallic catalysts for inorganic chemists. However, many homogeneous catalysts are non organometallic, as demonstrated by the use of cobalt salts that catalyze the oxidation of p-xylene to terephthalic acrid.
Whereas in the study of catalysis, transition metals ofttimes attract much of the focus, pocket-sized organic molecules without metals may also exhibit catalytic properties, as is axiomatic from the lack of transition metals in many enzymes. Organic catalysts normally demand a college load (catalyst quantity per unit quantity of reactant, expressed in mol percentage of substance) than transition metal(-ion)-based catalysts, but these catalysts are more often than not commercially available in bulk, helping to reduce costs. Such organocatalysts were considered a "new breed" in the early 2000s and are competitive with conventional catalysts containing metallic(-ion).
Case 1: Hydrolysis of ethyl acetate in the presence of dilute acid.
Ethyl acetate is a liquid that contains an ester functional group. Information technology reacts with water in the presence of dilute sulphuric acid which is a liquid to give ethyl alcohol and acetic acid.
\(\begin{array}{l}C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+{{H}_{2}}OCl \overset{H\oplus Cl} \longrightarrow C{{H}_{iii}}COOHCl+{{C}_{2}}{{H}_{5}}OHCl\cease{array} \)
In the above reaction reactants and catalysts are in the same country of matter. Hence, it is homogeneous catalysis
Example 2: Oxidation of sulphur dioxide in the pb chamber process.
Atomic number 82 chamber process is used in the manufacture of sulphuric acid. In this process, Nitric oxide gas is used as catalysis.
\(\begin{array}{l}2S{{O}_{ii}}\left( thousand \correct)+{{O}_{2}}\left( g\right) \overset{NO(g)} \longrightarrow 2S{{O}_{3}}\left( g \correct)\end{array} \)
In the to a higher place reaction, And so2 and Oii along with catalyst NO is also a gas hence it is homogeneous catalysis.
Also Read: Wilkinson'southward Catalyst
Mechanism of Homogeneous Catalysis
The homogeneous catalysis takes place by intermediate compound formatter theory.
Permit the states consider the oxidation of And so2 into SO3 by the atomic number 82 sleeping room process. In this nitric oxide gas is the catalyst.
This NO reacts with And then2 to course SOii and "NO2" equally an intermediate compound.
\(\begin{assortment}{fifty}2S{{O}_{ii}}\left( 1000 \correct)+{{O}_{2}}\left( k \correct) \overset{NO\left( g \correct)} \longrightarrow 2S{{O}_{3}}\left( g \right)\end{array} \)
Commencement step: Nitric oxide combines with oxygen to form nitrogen dioxide (NO2). This NO2 acts as an intermediate chemical compound, which reacts with So2 to form sulphur trioxide and NO
2NO(g) + Oii(1000) → 2NO2(g) Intermediate compound
2SO2 + 2NOtwo → 2SO3(grand) + 2NO(g)
Photocatalysts
Photocatalysis is the phenomenon wherein the catalyst is able to receive low-cal (such every bit visible calorie-free) and exist promoted to an excited state.
Autocatalysis
In the autocatalytic reaction, there is no specific goad that is added. Instead, one of the products acts as a catalyst and increases the rate of formation of products.
Case 1: Decomposition of Arsene (AsHthree) is formed by the Arsenic formed in the reactor is "autocatalyst".
2As H3 → 2As + 3H2
In this process As acts equally a goad.
Example 2: Oxidation of Oxalic acrid past KMnO4
When Permanganate is added to acidic solution oxidation of oxalate ions (or oxalic acrid) occur. The reaction results in the formation of car-catalyzes the reaction. The rate of reaction between Potassium permanganate and acidified oxalate solution is initially slow. ions that are formed during the reaction helps in increasing the charge per unit of reaction.
Frequently Asked Questions On Catalyst
How tin a positive catalyst alter the reaction?
A positive catalyst is to make reaction rate very first by changing the path of reaction by decreasing the activation energy basis. Such that a large number of reactant molecules are converted into products.
What is the part of catalyst toxicant in Rosenmund reaction?
In the Rosenmund reaction aldehyde is prepared past reducing Acrid halides with hydrogen gas in the presence of palladium. If a catalyst is not poisoned the reaction is not stopped at aldehyde level which is feather reduces of booze. In gild to end at the aldehyde level. Palladium is poisoned with Barium sulphate.
What are the central factors in heterogeneous catalysis?
In heterogeneous catalysis, the reacting and goad are in different states of thing. The nearly of import steps in this process are;
– Adsorption of reactant molecules activation centre.
– Formation of activation circuitous at the centre.
– This complex decomposes to give products.
– Desorption of products from the surface of the catalyst.
What is the role of promoters in Haber's process?
Promotors or accelerators increase the catalyst activity in a process. In Haber'due south process of manufacturing Ammonia, Nitrogen reacts with hydrogen to form NH3. Nitrogen is very less reactive and the yield of Ammonia is very less, to increase the percentage yield of Ammonia formed NO is used equally a promoter.
What is the significance of autocatalysis?
Auto catalysis is self-catalysis in this process one of the products formed acts as a catalyst and increases the reaction charge per unit.
Act As A Catalyst Meaning,
Source: https://byjus.com/jee/catalyst/
Posted by: moorehicave.blogspot.com


0 Response to "Act As A Catalyst Meaning"
Post a Comment