Lewis Structure For Polyatomic Ions
x.5: Writing Lewis Structures for Covalent Compounds
- Page ID
- 47529
↵
- Draw Lewis structures for covalent compounds.
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions.
1. Determine the total number of valence electrons in the molecule or ion.
- Add together together the valence electrons from each atom. (Recall that the number of valence electrons is indicated by the position of the element in the periodic tabular array.)
- If the species is a polyatomic ion, remember to add or subtract the number of electrons necessary to requite the total accuse on the ion.
For COiii 2 −, for example, we add two electrons to the total because of the −two charge.
ii. Arrange the atoms to show specific connections.
- When there is a central cantlet, it is usually the least electronegative element in the chemical compound. Chemists usually listing this primal cantlet first in the chemical formula (as in CCl4 and COiii 2 −, which both have C as the central cantlet), which is another clue to the compound's structure.
- Hydrogen and the halogens are near e'er connected to only one other atom, so they are usually concluding rather than central.
3. Identify a bonding pair of electrons between each pair of next atoms to give a single bond.
- In H2O, for example, there is a bonding pair of electrons between oxygen and each hydrogen.
iv. Kickoff with the terminal atoms, add enough electrons to each atom to requite each atom an octet (two for hydrogen).
- These electrons volition normally be lonely pairs.
v. If any electrons are left over, identify them on the central atom.
- We will explicate later that some atoms are able to accommodate more than than eight electrons.
six. If the central cantlet has fewer electrons than an octet, utilize lone pairs from terminal atoms to grade multiple (double or triple) bonds to the cardinal atom to achieve an octet.
- This will not change the number of electrons on the last atoms.
seven. Final check
- Always make sure all valence electrons are accounted for and that each cantlet has an octet of electrons, except for hydrogen (with ii electrons).
- The central cantlet is usually the least electronegative chemical element in the molecule or ion; hydrogen and the halogens are usually last.
Now permit's utilise this procedure to some detail compounds, beginning with i we take already discussed.
Write the Lewis Construction for H2O.
Solution
| Steps for Writing Lewis Structures | Instance \(\PageIndex{1}\) |
|---|---|
| 1. Make up one's mind the total number of valence electrons in the molecule or ion. | Each H atom (group ane) has 1 valence electron, and the O atom (grouping xvi) has 6 valence electrons, for a total of viii valence electrons. |
| 2. Arrange the atoms to evidence specific connections. | H O HBecause H atoms are virtually always terminal, the arrangement within the molecule must exist HOH. |
| three. Place a bonding pair of electrons between each pair of adjacent atoms to give a unmarried bail. four. Beginning with the concluding atoms, add plenty electrons to each atom to give each atom an octet (two for hydrogen). | Placing ane bonding pair of electrons between the O cantlet and each H atom gives H -O- Hwith 4 electrons left over. Each H atom has a full valence shell of 2 electrons. |
| five. If whatever electrons are left over, place them on the central atom. | Adding the remaining 4 electrons to the oxygen (as 2 lone pairs) gives the following structure: |
| six. If the central atom has fewer electrons than an octet, use lonely pairs from terminal atoms to form multiple (double or triple) bonds to the cardinal atom to reach an octet. | Non necessary. |
| 7. Last bank check. | The Lewis structure gives oxygen an octet and each hydrogen two electrons. |
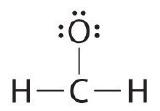
Write the Lewis structure for the \(CH_2O\) molecule
Solution
| Steps for Writing Lewis Structures | Example \(\PageIndex{2}\) |
|---|---|
| 1. Make up one's mind the total number of valence electrons in the molecule or ion. | Each hydrogen atom (group 1) has 1 valence electron, carbon (grouping 14) has four valence electrons, and oxygen (group 16) has 6 valence electrons, for a full of [(2)(1) + 4 + 6] = 12 valence electrons. |
| 2. Arrange the atoms to show specific connections. | Because carbon is less electronegative than oxygen and hydrogen is commonly terminal, C must exist the fundamental cantlet. |
| iii. Place a bonding pair of electrons betwixt each pair of side by side atoms to give a unmarried bond. | Placing a bonding pair of electrons betwixt each pair of bonded atoms gives the following: six electrons are used, and six are left over. |
| 4. Showtime with the terminal atoms, add enough electrons to each cantlet to give each atom an octet (two for hydrogen). | Adding all 6 remaining electrons to oxygen (as three lone pairs) gives the post-obit: Although oxygen at present has an octet and each hydrogen has ii electrons, carbon has only 6 electrons. |
| 5. If any electrons are left over, identify them on the cardinal atom. | Non necessary. At that place are no electrons left to place on the central atom. |
| 6. If the central atom has fewer electrons than an octet, use lone pairs from terminal atoms to form multiple (double or triple) bonds to the cardinal atom to achieve an octet. | To requite carbon an octet of electrons, we utilize one of the lonely pairs of electrons on oxygen to form a carbon–oxygen double bond: |
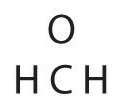
| seven. Final check | Both the oxygen and the carbon at present have an octet of electrons, so this is an adequate Lewis electron structure. The O has ii bonding pairs and two alone pairs, and C has four bonding pairs. This is the structure of formaldehyde, which is used in embalming fluid. |
Write Lewis electron structures for CO2 and SCl2, a vile-smelling, unstable red liquid that is used in the manufacture of rubber.
- Answer CO2
-
.


- Reply SCltwo
-
.
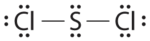
The United states Supreme Court has the unenviable job of deciding what the law is. This responsibility tin can be a major challenge when there is no articulate principle involved or where there is a new situation not encountered before. Chemical science faces the same claiming in extending basic concepts to fit a new situation. Drawing of Lewis structures for polyatomic ions uses the same approach, merely tweaks the process a piddling to fit a somewhat different fix of circumstances.
Writing Lewis Structures for Polyatomic Ions (CK-12)
Recall that a polyatomic ion is a group of atoms that are covalently bonded together and which carry an overall electrical charge. The ammonium ion, \(\ce{NH_4^+}\), is formed when a hydrogen ion \(\left( \ce{H^+} \right)\) attaches to the lonely pair of an ammonia \(\left( \ce{NH_3} \right)\) molecule in a coordinate covalent bond.
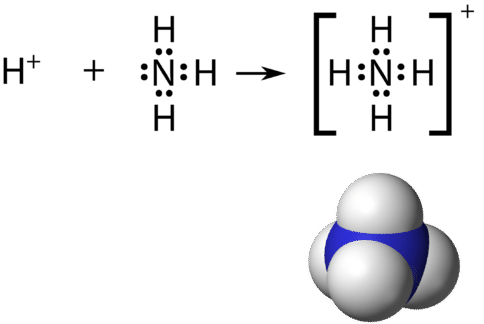
When drawing the Lewis construction of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion:
\(1 \: \ce{N}\) atom \(= v\) valence electrons
\(4 \: \ce{H}\) atoms \(= 4 \times i = 4\) valence electrons
subtract 1 electron for the \(1+\) charge of the ion
total of viii valence electrons in the ion
It is customary to put the Lewis structure of a polyatomic ion into a large set of brackets, with the charge of the ion every bit a superscript exterior of the brackets.
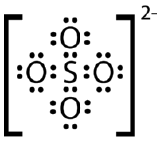
Draw the Lewis electron dot structure for the sulfate ion.
- Answer(CK12 License)
-
Exceptions to the Octet Dominion (BC Campus)
As important and useful as the octet rule is in chemical bonding, at that place are some well-known violations. This does not mean that the octet dominion is useless—quite the contrary. As with many rules, there are exceptions, or violations.
There are three violations to the octet dominion. Odd-electron molecules correspond the starting time violation to the octet rule. Although they are few, some stable compounds accept an odd number of electrons in their valence shells. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Examples of stable odd-electron molecules are NO, NO 2 , and ClO 2 . The Lewis electron dot diagram for NO is as follows:
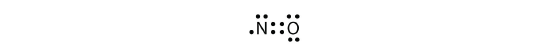
Although the O cantlet has an octet of electrons, the Northward atom has only seven electrons in its valence shell. Although NO is a stable compound, it is very chemically reactive, as are virtually other odd-electron compounds.
Electron-deficient molecules represent the second violation to the octet dominion. These stable compounds take less than eight electrons effectually an cantlet in the molecule. The virtually mutual examples are the covalent compounds of beryllium and boron. For case, beryllium can class two covalent bonds, resulting in only four electrons in its valence shell:
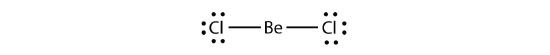
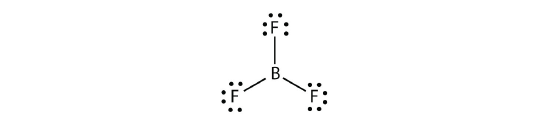
Boron usually makes but three covalent bonds, resulting in but six valence electrons effectually the B cantlet. A well-known example is BF three :
The third violation to the octet dominion is found in those compounds with more than 8 electrons assigned to their valence beat. These are called expanded valence vanquish molecules. Such compounds are formed merely past fundamental atoms in the third row of the periodic table or beyond that have empty d orbitals in their valence shells that tin participate in covalent bonding. 1 such compound is PF five . The only reasonable Lewis electron dot diagram for this chemical compound has the P atom making five covalent bonds:
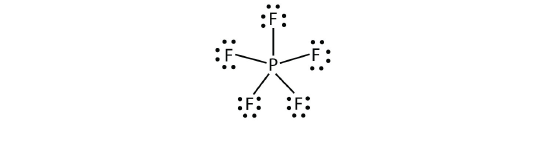
Formally, the P atom has 10 electrons in its valence shell.
Identify each violation to the octet rule by drawing a Lewis electron dot diagram.
- ClO
- SF half dozen
Solution
a. With one Cl atom and ane O atom, this molecule has vi + 7 = 13 valence electrons, so it is an odd-electron molecule. A Lewis electron dot diagram for this molecule is as follows:
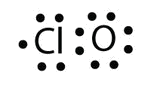
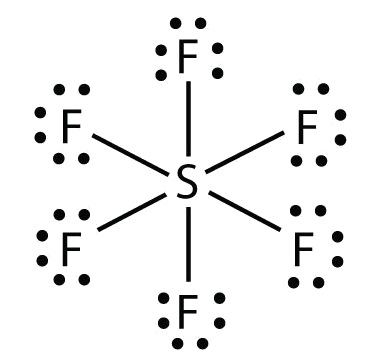
b. In SF 6 , the central S atom makes half-dozen covalent bonds to the half dozen surrounding F atoms, and so it is an expanded valence beat molecule. Its Lewis electron dot diagram is as follows:
Identify the violation to the octet rule in XeF 2 by cartoon a Lewis electron dot diagram.
- Answer
-
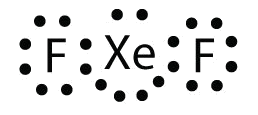
The Xe atom has an expanded valence crush with more than eight electrons around it.
Summary
Lewis dot symbols provide a simple rationalization of why elements form compounds with the observed stoichiometries. A plot of the overall energy of a covalent bond as a function of internuclear distance is identical to a plot of an ionic pair considering both outcome from attractive and repulsive forces between charged entities. In Lewis electron structures, we encounter bonding pairs, which are shared by ii atoms, and lonely pairs, which are not shared between atoms. Lewis structures for polyatomic ions follow the same rules as those for other covalent compounds. There are three violations to the octet dominion: odd-electron molecules, electron-deficient molecules, and expanded valence vanquish molecules.
Lewis Structure For Polyatomic Ions,
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/10%3A_Chemical_Bonding/10.05%3A_Writing_Lewis_Structures_for_Covalent_Compounds
Posted by: moorehicave.blogspot.com



0 Response to "Lewis Structure For Polyatomic Ions"
Post a Comment